Transition map analysis¶
[1]:
import cospar as cs
[2]:
cs.logging.print_version()
cs.settings.verbosity = 2 # range: 0 (error),1 (warning),2 (info),3 (hint).
cs.settings.set_figure_params(
format="png", figsize=[4, 3.5], dpi=75, fontsize=14, pointsize=3
)
Running cospar 0.2.0 (python 3.8.12) on 2022-02-08 19:23.
[3]:
# Each dataset should have its folder to avoid conflicts.
cs.settings.data_path = "data_cospar"
cs.settings.figure_path = "fig_cospar"
cs.hf.set_up_folders()
Load an existing dataset. (If you have pre-processed data, you can load it with cs.hf.read(file_name).)
[4]:
adata_orig = cs.datasets.hematopoiesis_subsampled()
Generate a transition map
[5]:
adata = cs.tmap.infer_Tmap_from_multitime_clones(
adata_orig,
clonal_time_points=["2", "4"],
later_time_point="6",
smooth_array=[20, 15, 10, 5],
sparsity_threshold=0.1,
intraclone_threshold=0.2,
max_iter_N=10,
epsilon_converge=0.01,
)
Trying to set attribute `.uns` of view, copying.
------Compute the full Similarity matrix if necessary------
------Infer transition map between initial time points and the later time one------
--------Current initial time point: 2--------
Step 1: Select time points
Number of multi-time clones post selection: 185
Step 2: Optimize the transition map recursively
Load pre-computed similarity matrix
Iteration 1, Use smooth_round=20
Iteration 2, Use smooth_round=15
Iteration 3, Use smooth_round=10
Iteration 4, Use smooth_round=5
Convergence (CoSpar, iter_N=4): corr(previous_T, current_T)=0.942
Iteration 5, Use smooth_round=5
Convergence (CoSpar, iter_N=5): corr(previous_T, current_T)=0.996
--------Current initial time point: 4--------
Step 1: Select time points
Number of multi-time clones post selection: 500
Step 2: Optimize the transition map recursively
Load pre-computed similarity matrix
Iteration 1, Use smooth_round=20
Iteration 2, Use smooth_round=15
Iteration 3, Use smooth_round=10
Iteration 4, Use smooth_round=5
Convergence (CoSpar, iter_N=4): corr(previous_T, current_T)=0.956
Iteration 5, Use smooth_round=5
Convergence (CoSpar, iter_N=5): corr(previous_T, current_T)=0.996
-----------Total used time: 19.324687957763672 s ------------
[6]:
# adata=cs.hf.read('data_cospar/LARRY_sp500_ranking1_MultiTimeClone_Later_FullSpace0_t*2*4*6_adata_with_transition_map.h5ad')
Key parameters¶
The analysis is done with the plotting module. There are some common parameters for the APIs in this module:
source (
str; default:transition_map). It determines which transition map to use for analysis. Choices: {transition_map,intraclone_transition_map,OT_transition_map,HighVar_transition_map,clonal_transition_map}selected_fates (
listofstr). Selected clusters to aggregate differentiation dynamics and visualize fate bias etc.. It allows nested structure, e.g., selected_fates=[‘a’, [‘b’, ‘c’]] selects two clusters: cluster ‘a’ and the other that combines ‘b’ and ‘c’.map_backward (
bool; default: True). We can analyze either the backward transitions, i.e., where these selected states or clusters came from (map_backward=True); or the forward transitions, i.e., where the selected states or clusters are going (map_backward=False).selected_times (
list; default: all). List of time points to use. By default, all are used.method (
str; default:norm-sum). Method to aggregate the transition probability within a cluster. Choices: {norm-sum,sum}.norm-sumreturns the probability that a fate cluster originates from an early state i; whilesumgives the probability that an initial state i gives rise to a fate cluster.
Plotting transition profiles for single cells¶
First, check the forward transitions (i.e., future states) from the 'transition_map'.
[7]:
selected_state_id_list = [
1,
10,
] # This is a relative ID. Its mapping to the actual cell id depends on map_backward.
map_backward = False
cs.pl.single_cell_transition(
adata,
selected_state_id_list=selected_state_id_list,
source="transition_map",
map_backward=map_backward,
)
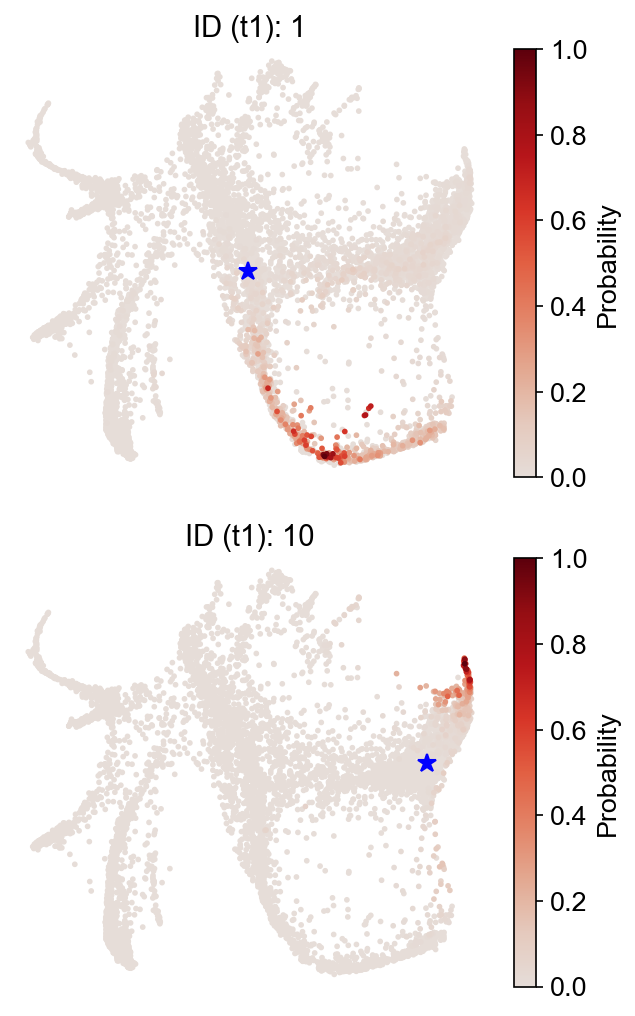
Now, backward transitions (i.e., past states) from the same map.
[8]:
selected_state_id_list = [2]
map_backward = True
cs.pl.single_cell_transition(
adata,
selected_state_id_list=selected_state_id_list,
source="transition_map",
map_backward=map_backward,
)
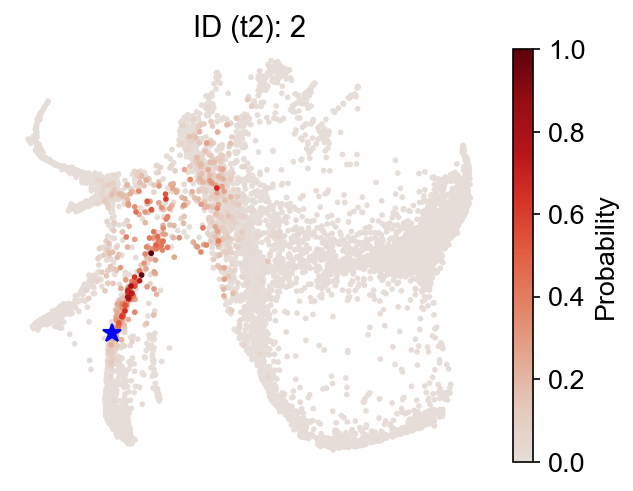
Finally, switch to the 'intraclone_transition_map', and check the observed clonal transitions:
[9]:
selected_state_id_list = [2]
map_backward = True
cs.pl.single_cell_transition(
adata,
selected_state_id_list=selected_state_id_list,
source="intraclone_transition_map",
map_backward=map_backward,
)
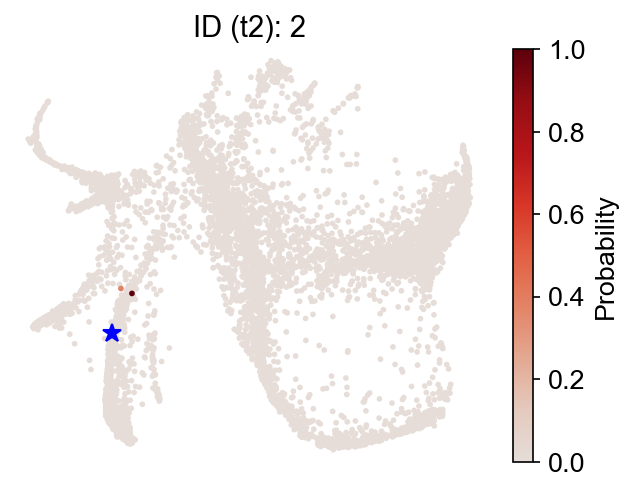
Fate map¶
Inspect the backward transitions, and ask where the selected fate clusters come from.
[10]:
cs.tl.fate_map(
adata,
selected_fates=["Neutrophil", "Monocyte"],
source="transition_map",
map_backward=True,
)
cs.pl.fate_map(
adata,
selected_fates=["Neutrophil"],
source="transition_map",
plot_target_state=True,
show_histogram=False,
)
Results saved at adata.obs['fate_map_transition_map_Neutrophil']
Results saved at adata.obs['fate_map_transition_map_Monocyte']
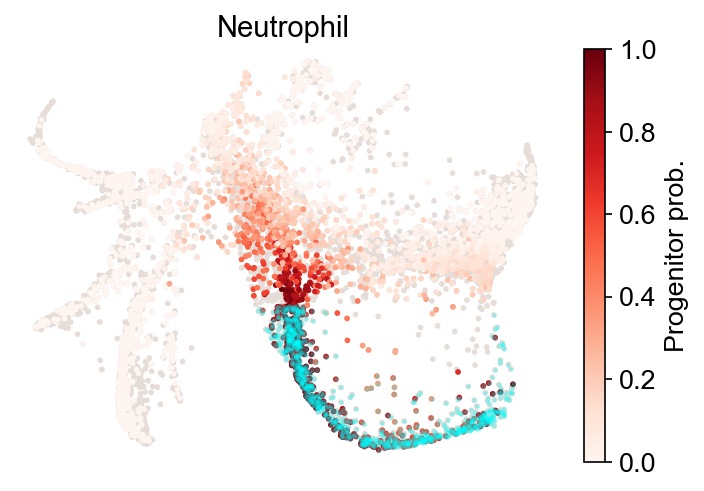
The results are stored at adata.obs[f'fate_map_{source}_{fate_name}'], which can be used for a customized analysis.
[11]:
adata.obs.keys()
[11]:
Index(['time_info', 'state_info', 'n_counts',
'fate_map_transition_map_Neutrophil',
'fate_map_transition_map_Monocyte'],
dtype='object')
As an example of how to use this data, you can re-plot the fate map as follows:
[12]:
cs.pl.embedding(adata, color="fate_map_transition_map_Neutrophil")
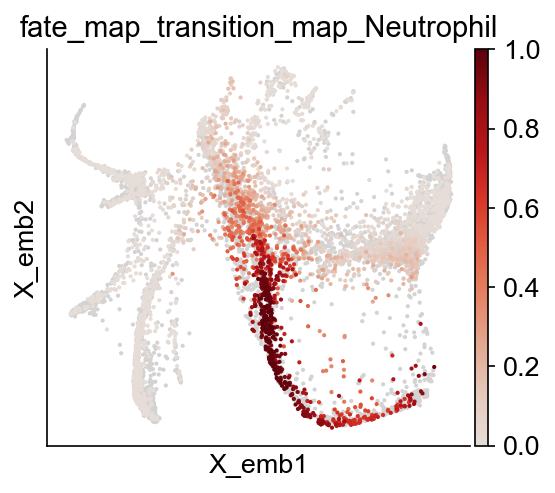
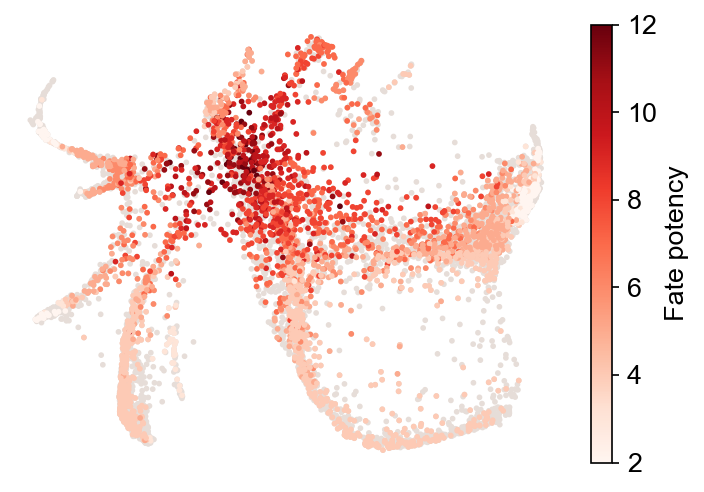
Fate potency¶
Count fate number directly (fate_count=True); otherwise, calculate the potencyusing entropy. The results are stored at adata.obs[f'fate_potency_{source}'].
[13]:
cs.tl.fate_potency(
adata,
source="transition_map",
map_backward=True,
method="norm-sum",
fate_count=True,
)
cs.pl.fate_potency(adata, source="transition_map")
Results saved at adata.obs['fate_map_transition_map_Ccr7_DC']
Results saved at adata.obs['fate_map_transition_map_Neu_Mon']
Results saved at adata.obs['fate_map_transition_map_undiff']
Results saved at adata.obs['fate_map_transition_map_Mast']
Results saved at adata.obs['fate_map_transition_map_Erythroid']
Results saved at adata.obs['fate_map_transition_map_Baso']
Results saved at adata.obs['fate_map_transition_map_Neutrophil']
Results saved at adata.obs['fate_map_transition_map_pDC']
Results saved at adata.obs['fate_map_transition_map_Lymphoid']
Results saved at adata.obs['fate_map_transition_map_Meg']
Results saved at adata.obs['fate_map_transition_map_Monocyte']
Results saved at adata.obs['fate_map_transition_map_Eos']
Results saved at adata.obs['fate_potency_transition_map']
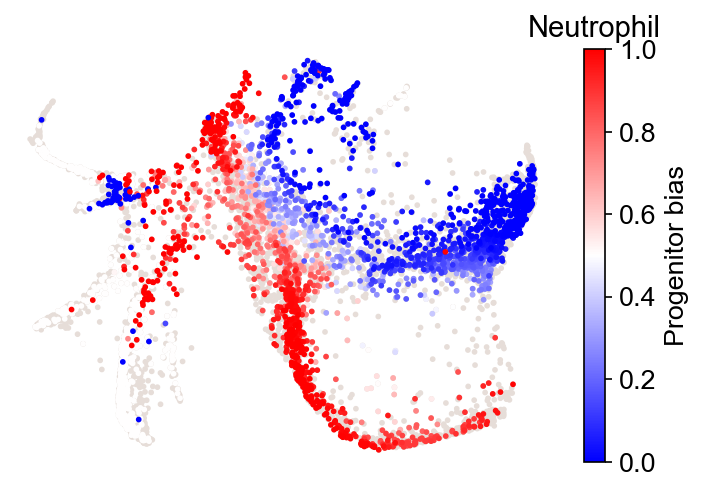
Fate bias¶
The fate bias of an initial state i is defined by the competition of fate probability towards two fate clusters A and B:
Bias_i=
P(i;A)/[P(i;A)+P(i;B)].
Only states with fate probabilities satisfying this criterion will be shown:
P(i; A)+P(i; B)>sum_fate_prob_thresh
The inferred fate bias is stored at adata.obs[f'fate_bias_{source}_{fate_A}*{fate_B}'].
[14]:
cs.tl.fate_bias(
adata,
selected_fates=["Neutrophil", "Monocyte"],
source="transition_map",
pseudo_count=0,
)
cs.pl.fate_bias(
adata,
selected_fates=["Neutrophil", "Monocyte"],
source="transition_map",
plot_target_state=False,
)
Results saved at adata.obs['fate_map_transition_map_Neutrophil']
Results saved at adata.obs['fate_map_transition_map_Monocyte']
Results saved at adata.obs['fate_bias_transition_map_Neutrophil*Monocyte']
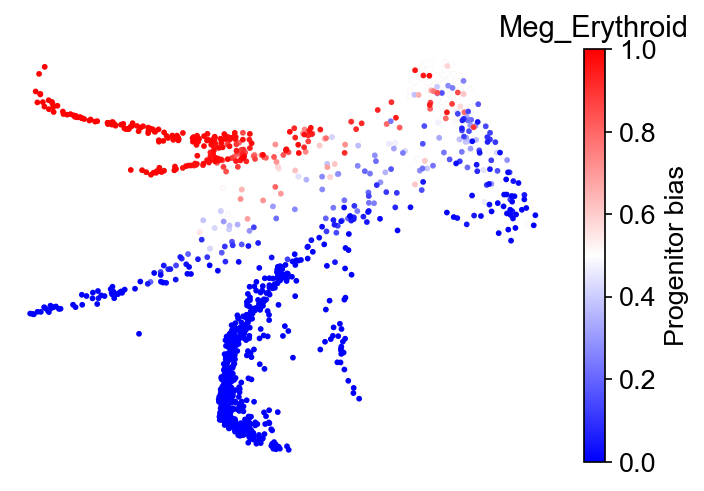
We can also study fate bias in the Gata1+ states. First, prepare a sate mask:
[15]:
x_emb = adata.obsm["X_emb"][:, 0]
y_emb = adata.obsm["X_emb"][:, 1]
index_2 = cs.hf.above_the_line(adata.obsm["X_emb"], [100, 500], [500, -1000])
index_5 = cs.hf.above_the_line(adata.obsm["X_emb"], [0, -500], [1000, 2])
final_mask = (~index_2) & (
(index_5 | (x_emb < 0))
) # & index_3 & index_4 & index_5 #mask_1 &
Now, compute the fate bias. We can concatenate cluster ‘Meg’ and ‘Erythroid’ using a nested list. Same for concatenating ‘Baso’, ‘Mast’, and ‘Eos’.
[16]:
# cs.pl.fate_bias(adata,selected_fates=[['Meg','Erythroid'],['Baso','Mast','Eos']],source='transition_map',
# plot_target_state=False,mask=final_mask,map_backward=True,sum_fate_prob_thresh=0.01,method='norm-sum')
cs.tl.fate_bias(
adata,
selected_fates=[["Meg", "Erythroid"], ["Baso", "Mast", "Eos"]],
source="transition_map",
pseudo_count=0,
map_backward=True,
sum_fate_prob_thresh=0.01,
method="norm-sum",
)
cs.pl.fate_bias(
adata,
selected_fates=[["Meg", "Erythroid"], ["Baso", "Mast", "Eos"]],
source="transition_map",
plot_target_state=False,
mask=final_mask,
background=False,
)
Results saved at adata.obs['fate_map_transition_map_Meg_Erythroid']
Results saved at adata.obs['fate_map_transition_map_Baso_Mast_Eos']
Results saved at adata.obs['fate_bias_transition_map_Meg_Erythroid*Baso_Mast_Eos']
Dynamic trajectory inference¶
We can infer the dynamic trajectory and ancestor population using the fate bias from binary fate competition. Here, fate bias is a scalar between (0,1) at each state. Selected ancestor population satisfies:
P(i;A) + P(i;B) > sum_fate_prob_thresh;
Ancestor states {i} for A: Bias_i > bias_threshold_A
Ancestor states {i} for B: Bias_i < bias_threshold_B
They will be stored at adata.obs[f'progenitor_{source}_{fate_name}'] and adata.obs[f'diff_trajectory_{source}_{fate_name}'].
[17]:
cs.tl.progenitor(
adata,
selected_fates=["Neutrophil", "Monocyte"],
source="transition_map",
map_backward=True,
bias_threshold_A=0.5,
bias_threshold_B=0.5,
sum_fate_prob_thresh=0.2,
avoid_target_states=True,
)
cs.pl.progenitor(
adata, selected_fates=["Neutrophil", "Monocyte"], source="transition_map"
)
Results saved at adata.obs['fate_map_transition_map_Neutrophil']
Results saved at adata.obs['fate_map_transition_map_Monocyte']
Results saved at adata.obs['fate_bias_transition_map_Neutrophil*Monocyte']
Results saved at adata.obs[f'progenitor_transition_map_Neutrophil'] and adata.obs[f'diff_trajectory_transition_map_Neutrophil']
Results saved at adata.obs[f'progenitor_transition_map_Monocyte'] and adata.obs[f'diff_trajectory_transition_map_Monocyte']
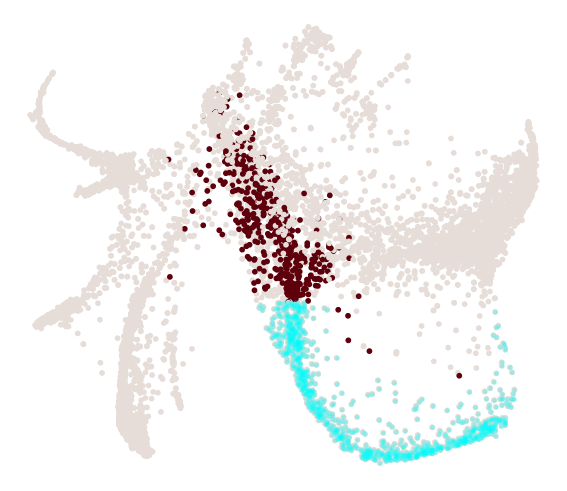
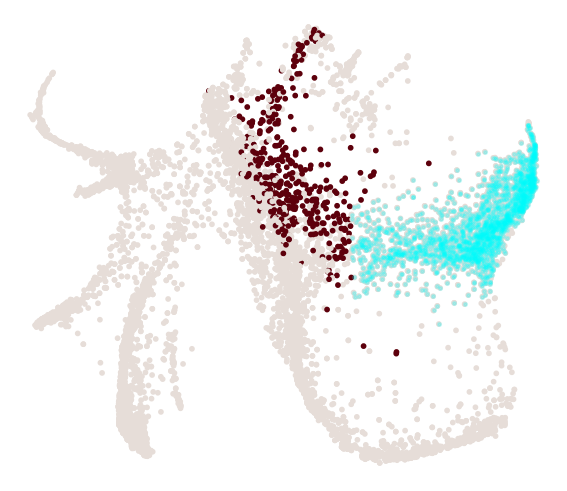
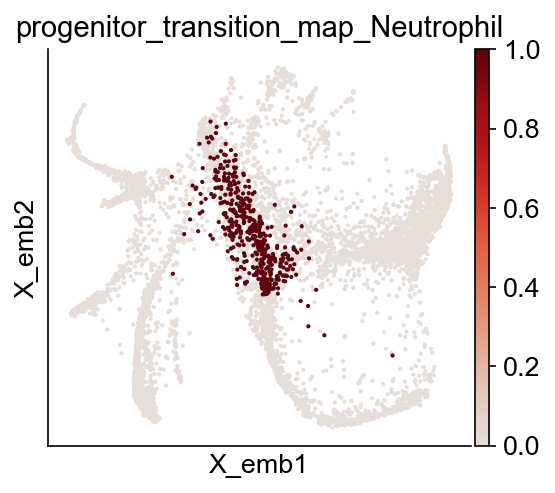
We can visualize the progenitors for Neutrophil directly
[18]:
fate_name = "Neutrophil" # Monocyte
traj_name = f"progenitor_transition_map_{fate_name}"
cs.pl.embedding(adata, color=traj_name)
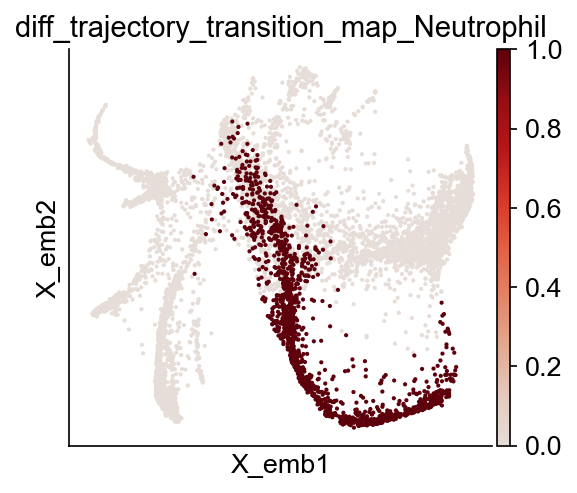
We can also visualize the differentiation trajectory for Neutrophil.
[19]:
fate_name = "Neutrophil" # Monocyte
traj_name = f"diff_trajectory_transition_map_{fate_name}"
cs.pl.embedding(adata, color=traj_name)
Differential genes for two ancestor groups¶
It would be interesting to see what genes are differentially expressed between these two ancestor populations, which might drive fate bifurcation. We provide a simple differentiation gene expression analysis that uses Wilcoxon rank-sum test to calculate P values, followed by Benjamini-Hochberg correction. You can always use your own method.
[20]:
import numpy as np
cell_group_A = np.array(adata.obs["diff_trajectory_transition_map_Neutrophil"])
cell_group_B = np.array(adata.obs["diff_trajectory_transition_map_Monocyte"])
dge_gene_A, dge_gene_B = cs.tl.differential_genes(
adata, cell_group_A=cell_group_A, cell_group_B=cell_group_B, FDR_cutoff=0.05
)
[21]:
dge_gene_A
[21]:
| index | gene | Qvalue | mean_1 | mean_2 | ratio | |
|---|---|---|---|---|---|---|
| 0 | 5 | Wfdc17 | 0.000000e+00 | 0.256167 | 24.515341 | -4.344265 |
| 1 | 9 | Lpl | 9.000377e-267 | 0.798759 | 27.861982 | -4.004097 |
| 2 | 0 | Mmp8 | 0.000000e+00 | 1.777057 | 40.070595 | -3.886477 |
| 3 | 304 | H2-Aa | 1.071471e-33 | 0.083807 | 14.977456 | -3.881858 |
| 4 | 8 | Ctss | 1.935638e-273 | 0.130347 | 15.299898 | -3.850025 |
| ... | ... | ... | ... | ... | ... | ... |
| 1061 | 1789 | H3f3a | 2.146904e-04 | 17.266771 | 18.038162 | -0.059673 |
| 1062 | 3329 | Gm37214 | 4.577627e-02 | 1.208660 | 1.298273 | -0.057379 |
| 1063 | 1881 | mt-Co2 | 3.645677e-04 | 195.855591 | 203.123032 | -0.052301 |
| 1064 | 2978 | Nom1 | 2.363414e-02 | 0.813894 | 0.820711 | -0.005412 |
| 1065 | 2647 | Ffar2 | 9.581307e-03 | 2.096291 | 2.100280 | -0.001857 |
1066 rows × 6 columns
Gene expression can be explored directly:
[22]:
selected_genes = dge_gene_A["gene"][:2]
cs.pl.gene_expression_on_manifold(
adata, selected_genes=selected_genes, color_bar=True, savefig=False
)
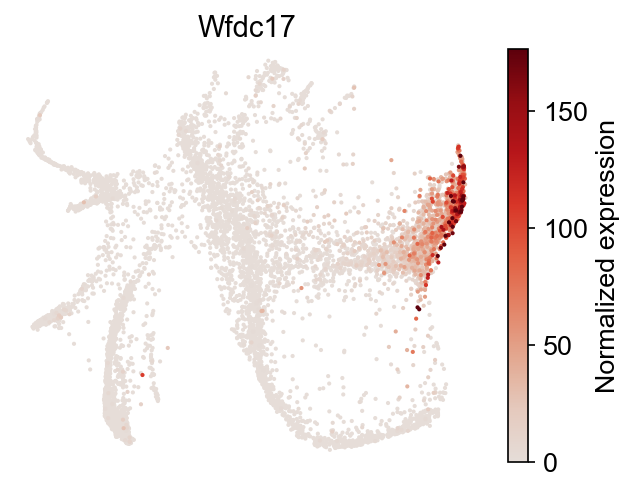
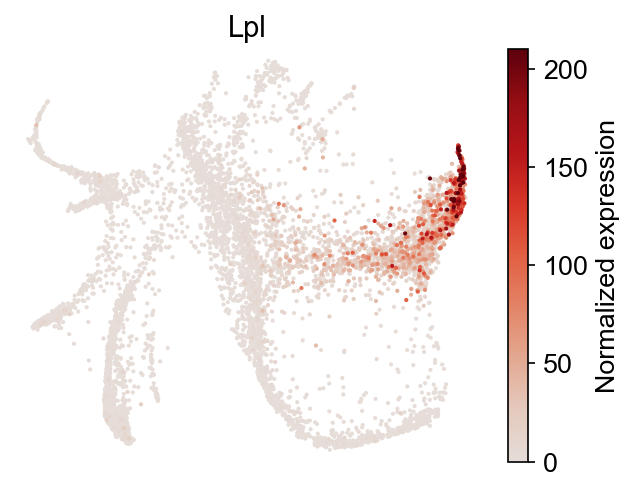
You can visualize the gene expression differences using a heat map.
[24]:
gene_list = list(dge_gene_A["gene"][:20]) + list(
dge_gene_B["gene"][:20]
) # select the top 20 genes from both populations
selected_fates = [
"Neutrophil",
"Monocyte",
["Baso", "Eos", "Erythroid", "Mast", "Meg"],
["pDC", "Ccr7_DC", "Lymphoid"],
]
renames = ["Neu", "Mon", "Meg-Ery-MBaE", "Lym-Dc"]
gene_expression_matrix = cs.pl.gene_expression_heatmap(
adata,
selected_genes=gene_list,
selected_fates=selected_fates,
rename_fates=renames,
fig_width=12,
)
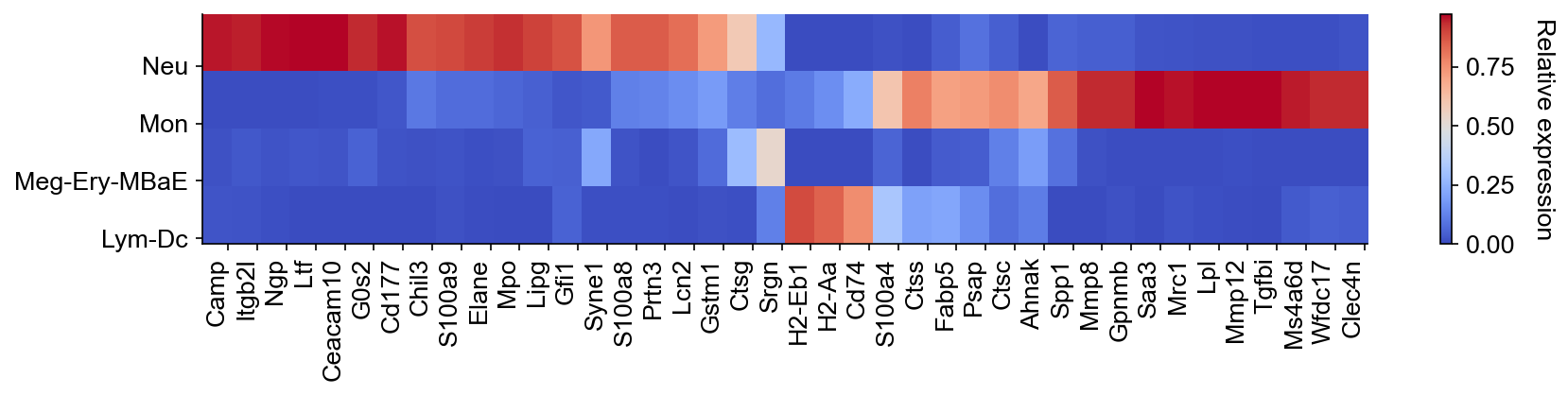
You can run the differential gene expression analysis on different cell clusters.
[25]:
dge_gene_A, dge_gene_B = cs.tl.differential_genes(
adata, cell_group_A="Neutrophil", cell_group_B="Monocyte"
)
Gene expression dynamics¶
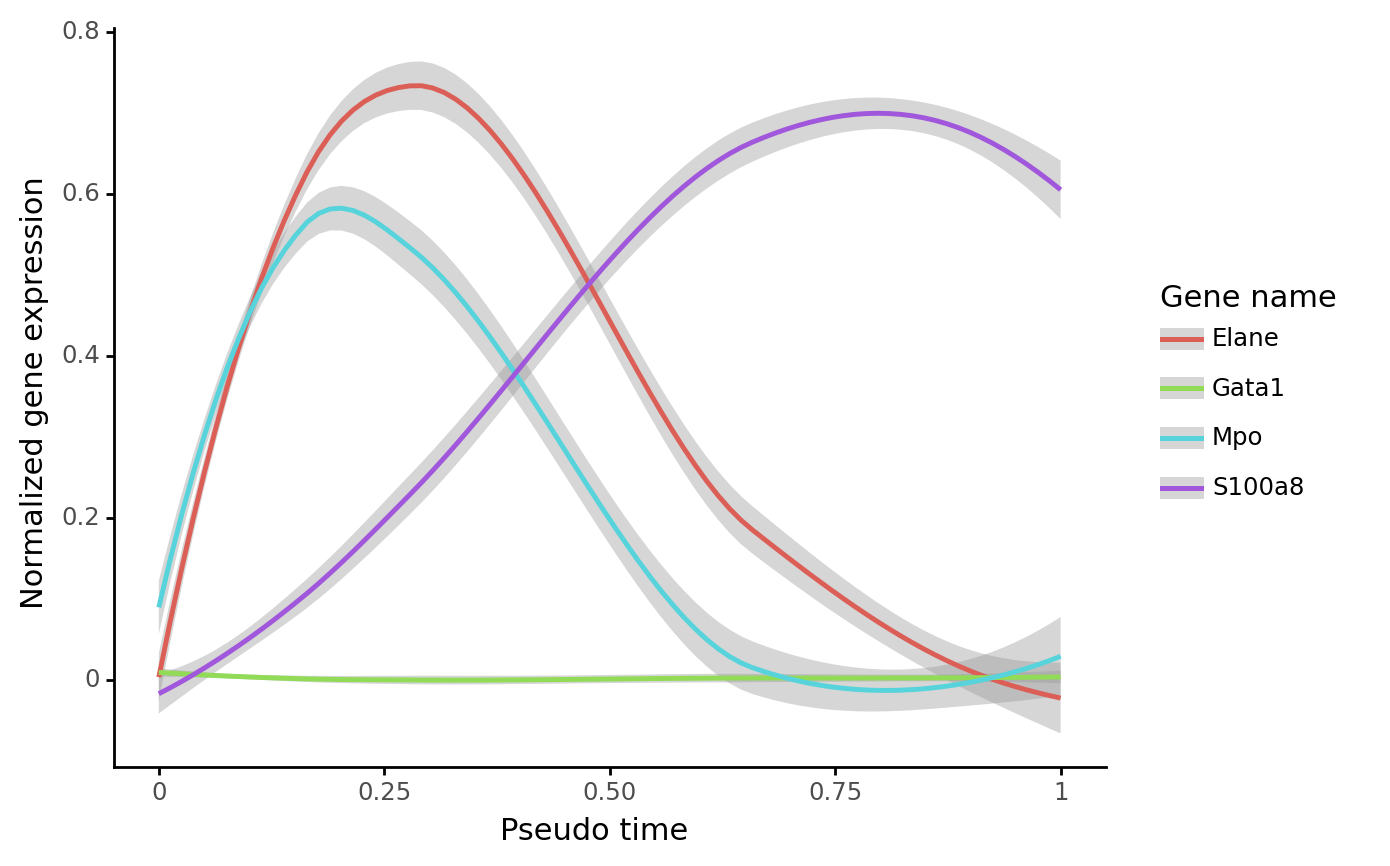
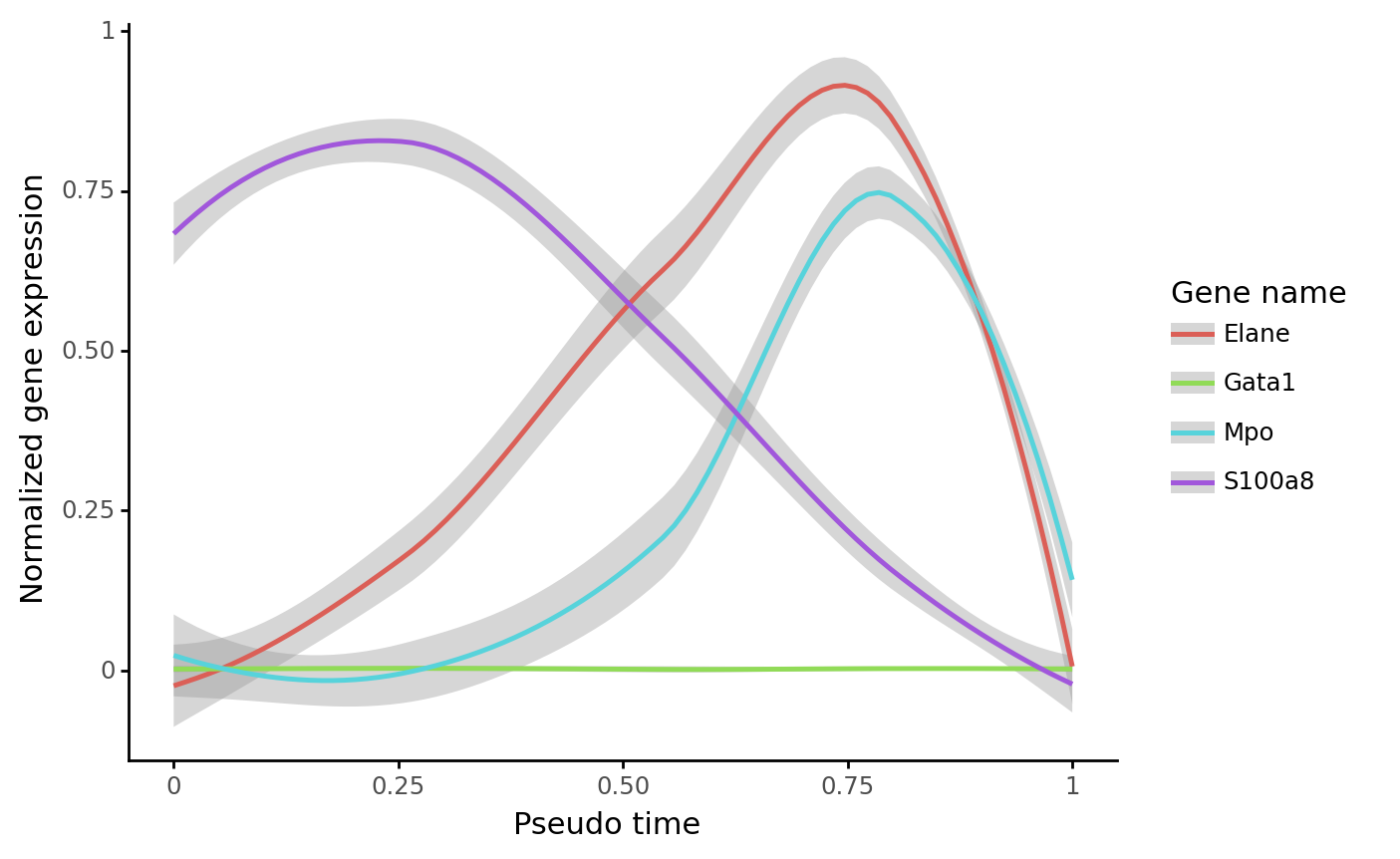
We can calculate the pseudotime along an inferred trajectory, and plot the gene expression along the pseudotime. This method requires that a trajectory has been inferred in previously steps.
[26]:
gene_name_list = ["Gata1", "Mpo", "Elane", "S100a8"]
selected_fate = "Neutrophil"
cs.pl.gene_expression_dynamics(
adata,
selected_fate,
gene_name_list,
traj_threshold=0.2,
invert_PseudoTime=False,
compute_new=True,
gene_exp_percentile=99,
n_neighbors=8,
plot_raw_data=False,
)
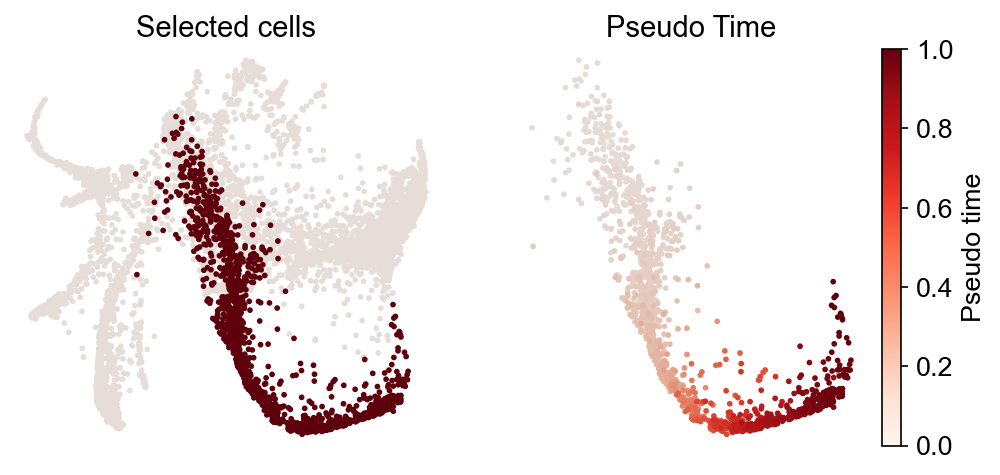
Fate coupling and hierarchy¶
The inferred transition map can be used to estimate differentiation coupling between different fate clusters.
[27]:
selected_fates = [
"Ccr7_DC",
"Mast",
"Meg",
"pDC",
"Eos",
"Lymphoid",
"Erythroid",
"Baso",
"Neutrophil",
"Monocyte",
]
cs.tl.fate_coupling(adata, selected_fates=selected_fates, source="transition_map")
cs.pl.fate_coupling(adata, source="transition_map")
Results saved as dictionary at adata.uns['fate_coupling_transition_map']
[27]:
<AxesSubplot:title={'center':'source: transition_map'}>
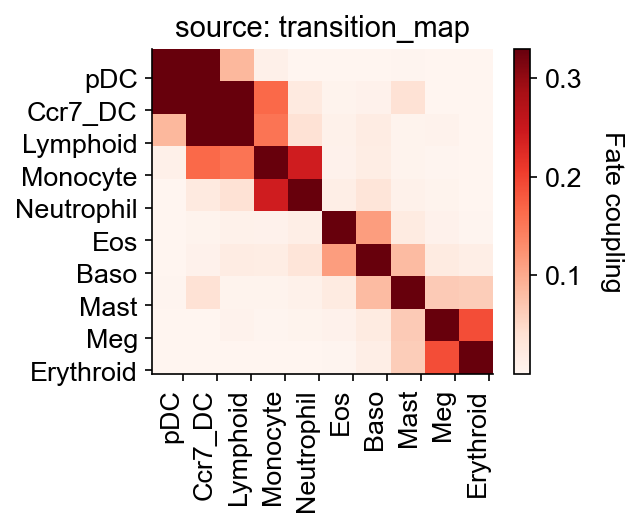
We can also infer fate hierarchy from a transition map, based on the fate coupling matrix and using the neighbor-joining method.
[28]:
cs.tl.fate_hierarchy(adata, selected_fates=selected_fates, source="transition_map")
cs.pl.fate_hierarchy(adata, source="transition_map")
Results saved as dictionary at adata.uns['fate_hierarchy_transition_map']
/-Baso
/-|
/-| \-Eos
| |
/-| \-Mast
| |
| | /-Erythroid
| \-|
--| \-Meg
|
| /-Monocyte
| /-|
| | \-Neutrophil
\-|
| /-Lymphoid
| /-|
\-| \-Ccr7_DC
|
\-pDC
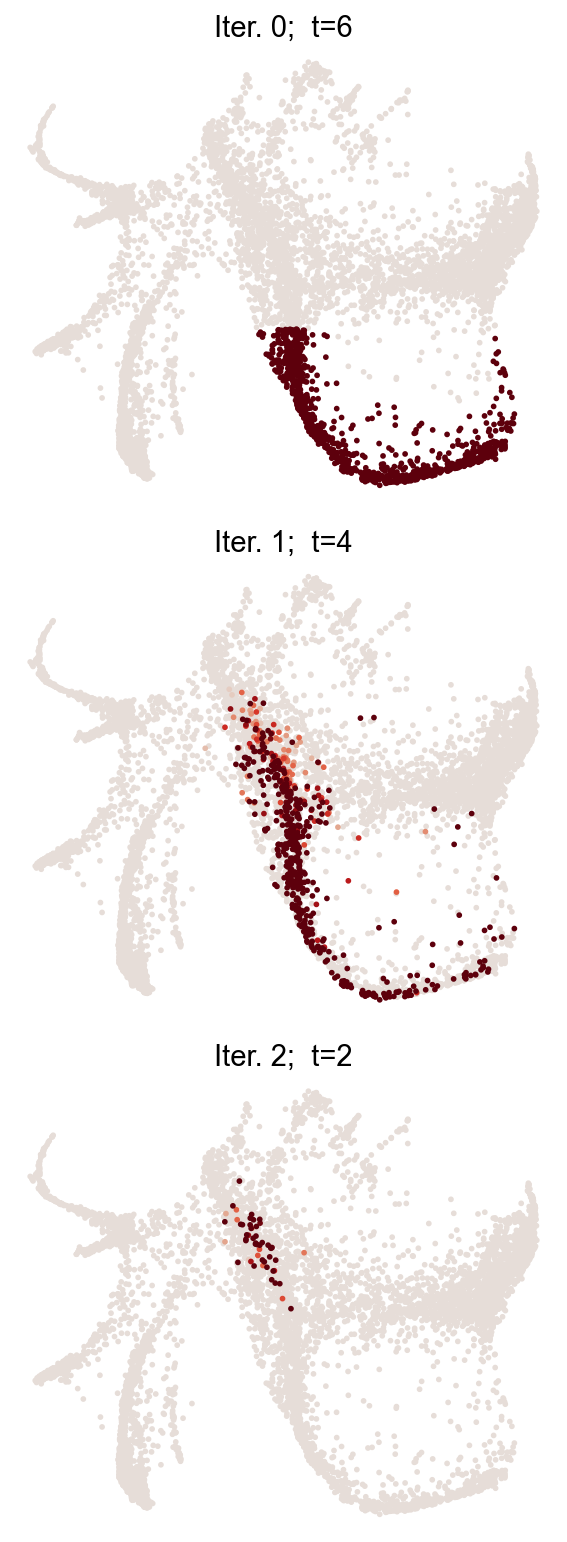
Propagate a cluster in time¶
One way to define the dynamic trajectory is simply mapping a given fate cluster backward in time. The whole trajectory across multiple time points will be saved at adata.obs[f'diff_trajectory_{source}_{fate_name}']. This method requires having multiple clonal time points, and a transition map between neighboring time points.
First, use all clonal time points (the default), and infer a transition map between neighboring time points (by not setting later_time_point).
[29]:
adata_5 = cs.tmap.infer_Tmap_from_multitime_clones(
adata_orig,
smooth_array=[20, 15, 10, 5],
sparsity_threshold=0.2,
intraclone_threshold=0.2,
max_iter_N=10,
epsilon_converge=0.01,
)
------Compute the full Similarity matrix if necessary------
----Infer transition map between neighboring time points-----
Step 1: Select time points
Number of multi-time clones post selection: 500
Step 2: Optimize the transition map recursively
Load pre-computed similarity matrix
Iteration 1, Use smooth_round=20
Iteration 2, Use smooth_round=15
Iteration 3, Use smooth_round=10
Iteration 4, Use smooth_round=5
Convergence (CoSpar, iter_N=4): corr(previous_T, current_T)=0.912
Iteration 5, Use smooth_round=5
Convergence (CoSpar, iter_N=5): corr(previous_T, current_T)=0.993
-----------Total used time: 17.11899995803833 s ------------
[30]:
cs.tl.iterative_differentiation(
adata_5, selected_fates="Neutrophil", source="intraclone_transition_map"
)
cs.pl.iterative_differentiation(adata_5, source="intraclone_transition_map")
Results saved at adata.obs[f'diff_trajectory_intraclone_transition_map_Neutrophil']
The trajectory can be used for visualizing gene expression dynamics using cs.pl.gene_expression_dynamics, as shown above:
[31]:
gene_name_list = ["Gata1", "Mpo", "Elane", "S100a8"]
selected_fate = "Neutrophil"
cs.pl.gene_expression_dynamics(
adata_5,
selected_fate,
gene_name_list,
traj_threshold=0.1,
invert_PseudoTime=True,
source="intraclone_transition_map",
)
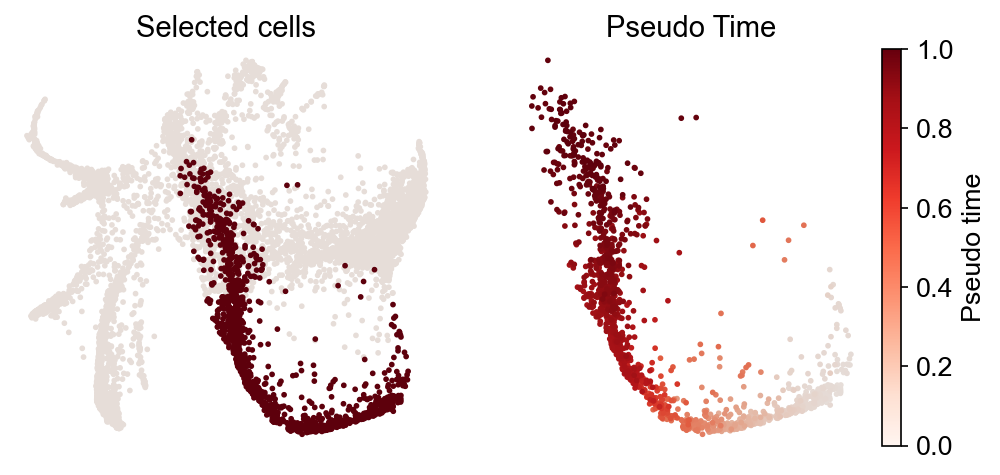
[ ]: